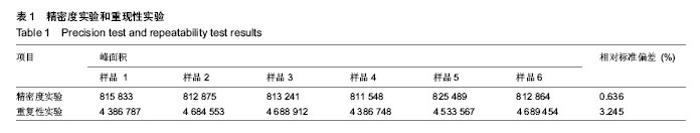
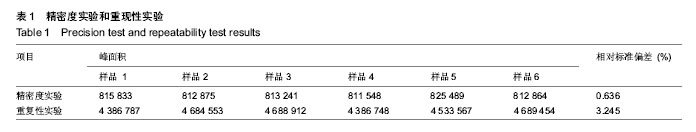
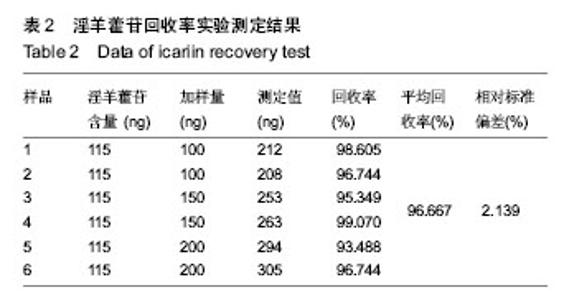
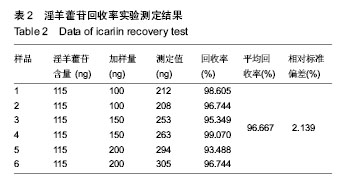
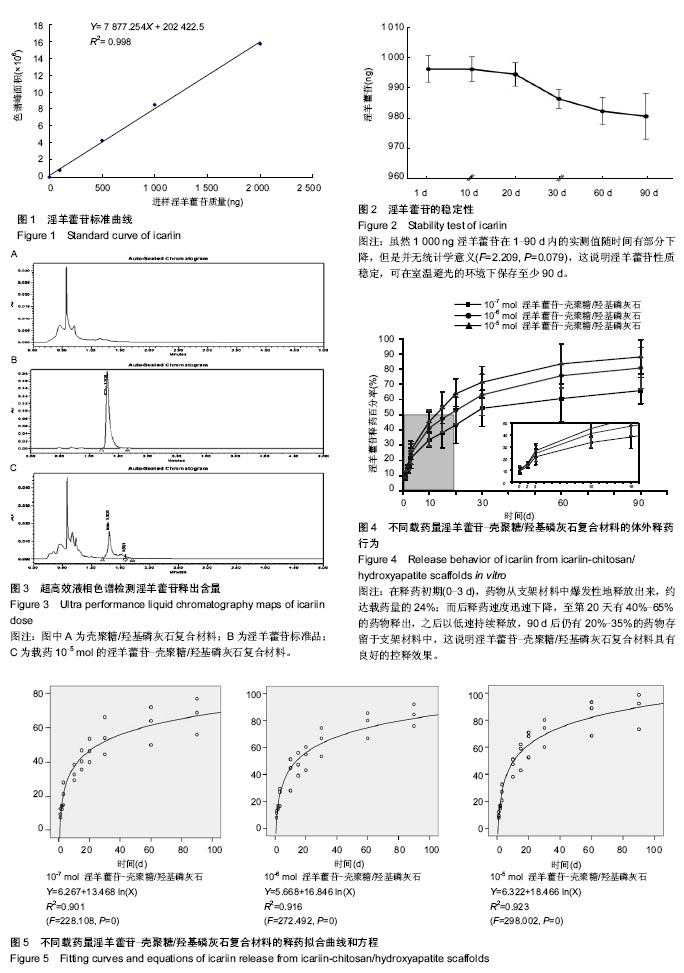
| [1] 吴涛,南开辉,金丹,等.仿生组装纳米羟基磷灰石/壳聚糖骨修复材料的制备及其生物相容性[J].中华创伤骨科杂志,2009,11(5): 4-28.
[2] Ruksudjarit A,Pengpat K,Rujijanagul G,et al.Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone.Curr Appl Phys. 2008;8(3-4):270-272.
[3] 张利,李玉宝,魏杰,等.纳米羟基磷灰石/壳聚糖复合骨修复材料的共沉淀法制备及其性能表征[J].功能材料,2005,36(3): 441-444.
[4] 王文良,张华亮,初殿伟,等.自体骨髓间充质干细胞复合壳聚糖/羟基磷灰石支架修复兔膝骨软骨缺损[J].中华骨科杂志, 2009, 29(1):61-64.
[5] 卢志华,马育栋,赵冬梅,等.羟基磷灰石/壳聚糖复合支架的制备及其性能研究[J].材料导报,2013,27(24):88-91.
[6] Ma XY,Feng YF,Ma ZS,et al.The promotion of osteointegraion under diabetic conditions using chitosan/ hydroxyapatitecomposite coating on porous titanium surfaces. Biomaterials.2014;35(26):7259-7270.
[7] Liu M,Zhong C,He RX,et al.Icariin associated with exercise therapy is an effective treatment for postmenopausal osteoporosis. Chin Med J.2012;125: 1784-1789.
[8] 吴涛,徐俊昌,南开辉,等.淫羊藿苷可促进羊骨髓间充质干细胞的增殖和成骨分化[J].中国组织工程研究与临床康复, 2009, 13(5): 3725-3729.
[9] Fan JJ,Cao LG,Wu T,et al.The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules. 2011;16(12): 10123-10133.
[10] Hsieh TP,Sheu SY,Sun JS,et al.Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine.2010;17(6):414.
[11] Cao H,Ke Y,Zhang Y,et al.Icariin stimulates MC3T3-E1 cell proliferation and differentiation through up-regulation of bone morphogenetic protein-2.Int J Mol Med.2012;29(3):435.
[12] 李会珍,李蒙,李瑞玉,等.淫羊藿对骨髓间充质干细胞成骨分化的影响[J].中国组织工程研究,2014,18(6):979-984.
[13] 朱鸿飞,郑军,徐小燕,等.不同浓度淫羊藿苷修复骨损伤:争议与探索[J].中国组织工程研究,2014,18(2):301-306.
[14] 李玲慧,丁道芳,杜国庆,等.淫羊藿苷对大鼠成骨细胞增殖及碱性磷酸酶、Runx2蛋白表达的影响[J].中国矫形外科杂志,2013, 21(21):17.
[15] Fan J,Bi L,Wu T,et al.A combined chitosan/nano-size hydroxyapatite system for the controlled release of icariin.J Mater Sci Mater Med.2012;23(2):399-407.
[16] 陈毅平,陈双英,陈文财,等.淫羊藿苷的稳定性及其影响因素[J].中国实验方剂学杂志, 2014,20(5):78-81.
[17] Wu T,Nna KH,Chen JD,et al.A new bone repair scaffold combined with chitosan/hydroxyapatite and sustained releasing icariin.Chin Sci Bull. 2009;54(17):2953-2961.
[18] 吴涛,南开辉,陈景帝,等.可控释淫羊藿苷的仿生组装骨诱导修复材料[J].科学通报,2009,54(9):1198-1206.
[19] Dash S,Murthy PN,Nath L,et al.Kinetic modeling on drug release from controlled drug delivery systems.Acta Pol Pharm.2010;67(3):217-223.
[20] Ji W,Sun Y,Yang F,et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications.Pharma Res. 2011;28(6):1259-1272.
[21] Yang Z,Wong CS,Yang C,et al.Control release effects of binders used in pills of traditional Chinese medicine herbs.Chem Pharm Bull.2006;54(2):188-195.
[22] Chen J,Lu WL,Gu W,et al.Drug-in-cyclodextrin-in-liposomes: a promising delivery system for hydrophobic drugs.Expert Opin Drug Deliv.2014;11(4):565-577.
[23] Venugopal J,Rajeswari R,Shayanti M,et al.Electrosprayed hydroxyapatite on polymer nanofibers to differentiate mesenchymal stem cells to osteogenesis.J Biomater Sci Polym Ed.2013;24(2):170-184.
[24] Strobel C,Bormann N,Kadow-Romacker A,et al.Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants.J Controll Release.2011;156(1): 37-45.
[25] Alman BA,Kelley SP,Nam D.Heal thyself: using endogenous regeneration to repair bone.Tissue Eng Part B Rev.2011; 17(6):431-436.
[26] Ko EC,Fujihara Y,Ogasawara T,et al.Administration of the insulin into the scaffold atelocollagen for tissue-engineered cartilage.J Biomed Mater Res A. 2011;97(2):186-192.
[27] Chen SH, Lei M, Xie XH, et al.PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits.Acta Biomaterialia.2013;9(5):6711-6722.
[28] Xie G, Sun J, Zhong G,et al. Hydroxyapatite nanoparticles as a controlled-release carrier of BMP-2: absorption and release kinetics in vitro. J Mater Sci Mater Med. 2010;21(6): 1875-1880.
[29] Maleki M,Amani-Tehran M,Latifi M,et al.Drug release profile in core-shell nanofibrous structures: a study on Peppas equation and artificial neural network modeling.Comput Methods Programs Biomed.2014;113(1):92-100.
[30] Qi R,Guo R,Zheng F,et al.Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly (lactic-co-glycolic acid) composite nanofibers. Colloids Surf B Biointerfaces.2013;110:148-155.
[31] 屠锡德,张钧寿,朱家璧.药剂学[M].3版.北京:人民卫生出版社, 2002.
[32] 钟大根.生物医用高分子材料在药物缓控释中的应用及其安全性评价[D].暨南大学, 2013.
[33] 杨义科,崔海燕.缓控释药物制剂数学模型的实例:改性扩散控制模型的建立及解析[J].许昌学院学报,2013,32(5):23-31.
[34] Ge Y,Mei Z,Liu X.Evaluation of daidzein-loaded chitosan microspheres in vivo after intramuscular injection in rats. Yakugaku Zasshi.2011;131(12):1807-1812. |